Morpheus Air Zero Hybrid System
The Morpheus Air Zero hybrid replacement mattress has been designed for users considered to be at 'High to Very High Risk' of developing pressure ulcers.
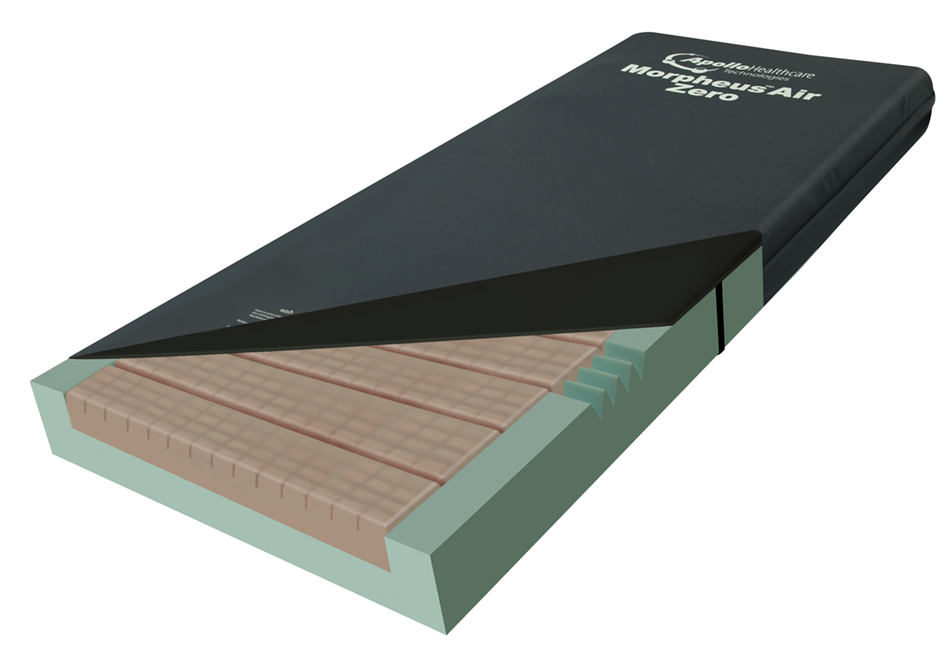
The Morpheus Air Zero is an Advance 4 Zoned Mattress Support System offering both Full Dynamic and Semi Dynamic Therapy.
The Morpheus Air Zero offers a therapeutic immersion support surface that simulates dynamic therapy whilst unpowered.
The Addition of a Pump converts the Mattress to Dynamic Mode when higher therapy is required.
The Morpheus Air Zero is suitable for all patient risks, offering reactive immersion therapy by means of innovative self-adjusting cell technology.
The Morpheus Air Zero behaves like a Dynamic System when unpowered, making it the mattress of choice for smokers when powered devices cannot be used.
- The mattress can be used as both a static support surface and a dynamic system
- Self-adjusting for all patient morphologies and positions
- Zoned for specific pressure redistribution and protection to vulnerable areas (4 Zoned Heel, Sacral, Torso and Head)
- Fully profiling flexible side walls
- Super-soft comfort layer on top of the core mattress
- Firm surrounding perimeter for easier patient mobilisation
- Static pillow section even when in powered mode
- Static use enables stable surface for safer patient transfers and handling
- Adjustable weight settings when used as a dynamic system
- Multi-stretch, breathable cover to contour to the patient and assist with microclimate moisture build up
- Smooth multi-stretch PU cover is both waterproof and vapour permeable, reducing shear and friction, and can be easily wiped clean or completely removed for laundering
- High frequency welded seams reduce the risk of fluid ingress, supporting infection control protocols
- The cover is clinically effective against bacteria/viruses, with proven resistance to MRSA
- Fire retardant to Standards BS7175 Crib 5
- Clear and simple controls reduces need for complex training
- Product Code - APH166Z
- Mattress Dimensions: 200(L) x 90(W) x 17(D) cm
- Mattress Weight: 17 kg
- Max User Weight: 39 stone / 248 kg
- Risk Category: High (Static) / Very High Risk (Dynamic)
-
Available as a Bariatric Option (Product Code - APH166Z/120)
Electrical
- Pump Dimensions: 28cm x 13.5cm x 9cm
- Pump Weight: 1.4 kg
- Pressure Range: 12 -26 mmHg
- Cycle Time: 10 minutes (1 in 2 cycle)
- Power Supply: AC 220v-240v 50Hz/60Hz 0.09A
- Noise Level: Below NC27
- Fuse: 500mA
- Medical Classification: Type BF Applied Part
- EMC Standards: BS EN 60601-1 BS5724-1 (CE , FDA , TUV)